The start of another flu season has found Americans facing a shortage of influenza vaccine. Although nearly 60 million flu shots will be available, vaccination is recommended for 185 million people at risk for complications. In a typical year, less than one-third of the U.S. population receives flu shots. Fortunately, since influenza is a contagious disease, it is not necessary to vaccinate everyone in order to stop widespread epidemics, or even to limit vaccinations only to those who are at risk. However, when there is a shortage, many people will seek the vaccine who might not otherwise have bothered.
How did this shortage come about? How can we prevent reoccurrences in the future?
The Demand for Flu Vaccines. Vaccination is one of the most beneficial and cost-effective health interventions. An estimated 65 million Americans catch the flu annually, resulting in 30 million physician visits. The U.S. Centers for Disease Control and Prevention (CDC) estimates the influenza virus contributes to the death of around 36,000 U.S. residents each year, and accounts for more than 200,000 hospitalizations. Yet, the past few years have found us without enough vaccine to serve all the population vulnerable to the worst affects of the disease: the elderly, the young and those with chronic medical problems.
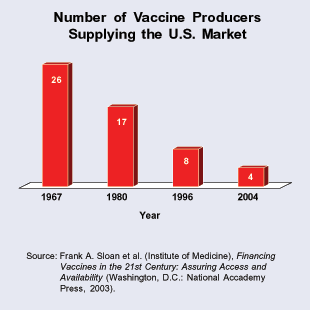
The Supply of Flu Vaccines. Nearly 40 years ago, there were about 26 makers of all types of vaccines. [See the figure.] The number fell to 17 by 1980. Now, the number of vaccine makers supplying the United States has dropped to only four. There is only a single manufacturer of the vaccines for eight diseases. There are only two major makers of flu vaccines. When there are so few producers of each vaccine, problems at a single plant can create shortages.
In recent years, the United States has come to depend on overseas vaccine plants to provide an increasing share of the supply. One company, the Chiron Corporation, prepared 46 million doses – almost half of the anticipated supply for the United States – at its plant in Liverpool, England. However, this year's production run was rejected for import by the U.S. Food and Drug Administration (FDA) because of contamination problems.
Furthermore, making a flu vaccine is a moving target. The CDC must estimate nearly a year in advance which strains of the flu making its way across China are likely to hit North America nine months later. The vaccine we buy is actually a cocktail of two or three different strains, each of which is incubated separately. Vaccine production is complex – it takes several months to incubate a batch of vaccine. If the wrong strains are picked, the product is essentially worthless. If a batch is contaminated, it is too late to produce more for the current flu season.
On the other hand, there is only about a three-month window of opportunity for producers to sell their flu vaccines for the entire year. Once a flu season has passed, remaining stockpiles are worthless. Last year one of the U.S. companies that manufacturers flu vaccine, Aventis Pasteur, had to discard 5 million of the 43 million doses it produced. Two years ago, 12 million doses of flu vaccine went unused.
Vaccine Shortages. Vaccines should be a moneymaker for drug firms. After all, nearly 300 million people in the United States alone are potential customers. One reason that there are so few producers is that the government is by far the largest customer. According to Paul Offit of Children's Hospital of Philadelphia, author of a forthcoming book on the vaccine industry, the United States has weathered nine shortages of six vaccines since 2000. The shortages were driven by government bulk purchasing.
In 1993 Hillary Clinton championed the Vaccines for Children program, under which the government uses its purchasing power to negotiate discounts for vaccines and distributes them to physicians and various health agencies for free or at a reduced price, essentially creating a children's vaccine entitlement. As a result, more than half the supply of childhood vaccines is purchased by government at deep discounts. Many firms have simply chosen to leave the market, given the low profit margins. Less than two percent of drug company revenue is derived from vaccines.
Like children's vaccines, more than half of flu vaccines are directly purchased by the government, or indirectly reimbursed by the government at discounted prices. In a market where government is the dominant buyer, or monopsonist, the price may be driven so low that many producers cannot earn a profit. The exposure to legal liability lowers profitability even more. Thus manufacturers have dropped out of the vaccine market.
Cause of Shortages: Excessive Tort Liability. There are several reasons why firms avoid the U.S. market. But they can be summed up with one word "profit." When tens of millions of people receive an injection of an approved drug, there are bound to be those who suffer adverse reactions. This might include those who merely react to the virus in the vaccine, or those who are allergic to the eggs in which the weakened viruses are grown. Although the benefit to society of vaccination programs far outweighs the cost, a small number of individuals will become very sick. The potential liability can easily erase all the profits earned by a vaccine manufacturer.
Estimates vary, but product liability lawsuits by people who have adverse reactions may account for much more than half of the cost of most vaccines. To remedy this problem, experts like Henry I. Miller, M.D., recommend that once a manufacturer meets FDA regulatory requirements for vaccine approval, it should not be liable for damages from approved use of its products. The few individual patients suffering serious injury could be compensated by the government, as is the case with children's vaccines. In 1986, Congress passed legislation creating a compensation fund and providing some liability protection for makers of childhood vaccines. However, these protections have eroded somewhat as lawyers tested the limits of "no fault" vaccine protection. No liability protection exists for adult vaccines. Last year the U.S. House of Representatives passed such legislation but the Senate did not.
Cause of Shortages: Overregulation. A number of reforms to provide relief from regulations have been proposed to increase the supply of vaccines.
A promising approach is reciprocal regulatory agency approvals, which would allow vaccines approved and used successfully in other developed countries to be sold in the United States. Currently, the FDA is responsible for inspecting and certifying plants and processes used to produce vaccines just as it is for drugs. Vaccines manufactured abroad that have not been produced under conditions approved by the FDA may not be sold in this country. While this is meant to protect the public, it also raises the cost and delays the introduction of potentially lifesaving vaccines. For example, according to Dr. Miller, former director of the FDA's Office of Biotechnology, Americans do not have access to a high-quality vaccination for meningitis C, although several are in widespread use in Europe and Canada, with a track record of 20 million doses administered. The adoption of common standards and a reciprocal regulatory approval process would benefit all of the countries involved by creating a wider market for their vaccine products, and benefit their citizens by giving them access to more sources of supply.
Conclusion.To have a viable vaccine industry, we will have to create the incentive for firms to produce them. The first and foremost incentive has to be the potential for profitability. For this to happen the government needs to get out of the business of buying vaccines. Congress also needs to create a safe harbor or otherwise limit the liability of firms that produce or administer an approved vaccine. In addition regulatory reforms are needed to permit vaccine makers to innovate and find the best way to produce vaccines.
Devon Herrick, Ph.D., is a senior fellow with the National Center for Policy Analysis.