Today's health consumers are taking a more active role in diagnosing and treating themselves. With new tools to assist them – from Health Savings Accounts (HSAs) that allow patients to control some their health care dollars to an unprecedented wealth of information on Internet Web sites – consumers are more empowered than ever to understand their health condition(s) and participate in decisions about their treatment. Consumers have long used over-the-counter (OTC) remedies – from aspirin to zinc – to treat symptoms…at least initially. Now these traditional self-treatments have been joined by new, potent medications that the U.S. Food and Drug Administration (FDA) has moved from prescription-only to OTC status. From pain relievers to allergy pills, once available only by prescription, some 89 medications are now on store shelves.
Consumers benefit from self-administered OTC medications with lower prices and easier access. However, rigid FDA rules lock many drugs that are readily accessible to consumers in other countries behind U.S. pharmacy counters.
Drugs Switched from Prescription to Over the Counter. The FDA can approve a prescription drug for OTC sale once it decides that the benefits outweigh the risks, the potential for abuse is low, consumers can self-diagnose the condition, labels can be easily understood, and administration by health practitioners is unnecessary. The 89 prescription products the FDA has switched over the past 30 years include such familiar brands as Advil, Afrin, Drixoral, Aleve, Pepcid AC, Zantac-75, Nicorrette, Rogaine and Lamisil. Recently, however, FDA approval for OTC sale has slowed dramatically; in the past five years, it has reclassified only seven prescription drugs (including the bestselling drugs Claritin and Prilosec). Over a 20 year period, by contrast, European countries approved about four times as many prescription medications for OTC sale as the United States, according to a 2003 report from the Tufts Center for the Study of Drug Development.
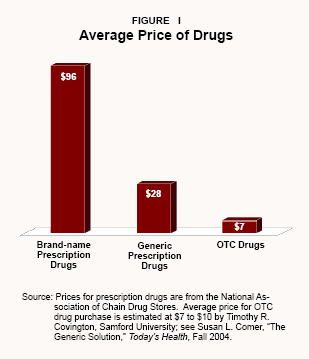
Prescription versus OTC Drug Prices . Every year, Americans spend more than $200 billion on prescription medicines, but only one-tenth that amount – about $20 billion – on OTC medications. Moreover, the average price of an OTC medication is much lower than that of a prescription drug. As Figure I shows:
- The average name-brand prescription costs about $96.
- Generic prescription drugs cost about $28.
- OTC drugs cost an average of about $7.
Research and development account for much of the higher cost of prescription medications, and average more than $800 million per drug. When drug patents expire, competing manufacturers are quick to produce lower-price generic versions. Prices fall even lower when a drug is switched to OTC status. For example, after the FDA approved the popular heartburn medication Prilosec for OTC sale in 2004, the price of a one-month supply dropped by more than 80 percent, from $132.90 to $21.49.
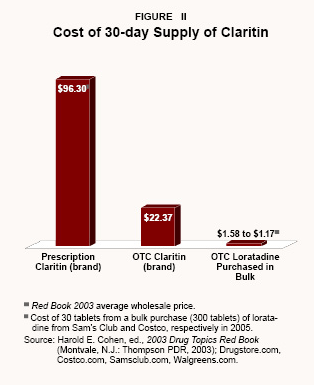
Similarly, a 30-day supply of the prescription allergy drug Claritin was available for $96.30 per month in 2003. [See Figure II.] But since the FDA approved an OTC version, a month's supply of OTC Claritin is available for $22.37, and generic OTC versions with the same active ingredient (loratadine) sell for less than $10 – and as low as $1.58 to $1.17 at some wholesale clubs.
Self-Care versus Professional Care. Surveys show that consumers often prefer to treat themselves. According to the Consumer Healthcare Products Association:
- About 73 percent of consumers would rather treat themselves than see a doctor.
- A majority (62 percent) say they would like to self-treat more in the future.
- Nearly all (96 percent) say they are generally confident about their health care decisions.
Most patients can self-administer OTC drugs safely. A 2001 survey by Roper Starch Worldwide indicates that most individuals take necessary precautions, such as "reading directions before taking a nonprescription product for the first time (95 percent), reading labels to choose appropriate OTC medicines (89 percent), and reading about possible side effects and interactions (91 percent)."
Furthermore, self-administered therapy for chronic conditions can be just as effective as professional care. For example, one study found that outcomes are about the same whether patients monitor their own blood pressure or rely solely on health professionals; but self-monitoring patients have 27 percent fewer physician visits. Asthma self-monitoring and telemonitoring (sending self-monitored results to a physician) have favorable results in 87 percent of cases. Spirometry (air flow) self-testing results are similar to spirometry supervised by a health professional. Glucose self-monitoring for diabetes has been very successful, and one study found that self-monitoring patients with bleeding disorders taking Warfarin had better outcomes than those who relied solely on health practitioners.
Patients with one or more of five chronic conditions (mood disorders, diabetes, heart disease, high blood pressure and asthma) account for almost half of all health care spending, according to Yale University research. If the FDA were to make drugs available over the counter to treat such chronic conditions as high cholesterol and heart disease, consumers would realize significant savings.
Conclusion: Increasing Opportunities for Consumers. Stringent FDA rules limit consumer access to treatments that could safely be moved over the counter. One solution is to allow any drug approved for OTC use in Europe to be sold in the United States. Another solution is to create a third class of drugs: behind-the-counter drugs that require consultation with the pharmacist rather than a doctor. Though these drugs could be purchased more conveniently and less expensive than prescription drugs, they would likely be more expensive (and less convenient) than unrestricted OTC drugs. Furthermore, a risk-adverse FDA could move unrestricted OTC drugs to more-restrictive behind the counter sale – making it more difficult for patients to self-treat.
Instead, FDA reforms should simplify and speed the process of switching today's prescription drugs to OTC status. Consumers would save billions of dollars, see improved access to beneficial therapies and assure them greater control of their own health care.
Paul Kittinger is an intern and Devon Herrick is a senior fellow with the National Center for Policy Analysis.