American hospitals and physicians are facing an unprecedented shortage of commonly used drugs. President Obama announced his support for legislation to address this problem by requiring drug makers to notify the Food and Drug Administration (FDA) of possible shortages six months in advance.1
The president also signed an executive order directing the FDA to streamline the process of approving changes to production lines and giving the Justice Department authority to investigate alleged price gouging.2 However, these steps will not fix the drug shortage problem and could even make it worse.
There are many reasons for drug shortages. Shortages of certain drugs reoccur due to a lack of competition and manufacturing problems. According to former White House adviser and oncologist Ezekiel Emanuel, only about 10 percent of shortages are due to a lack of raw materials needed to manufacture them. 3 A more important cause is government pricing policy.
The Drug Shortage Problem Is Real. Drug shortages are widespread. In a recent survey, nine-in-10 anesthesiologists reported experiencing a shortage of at least one anesthesia drug.4 Oncologists also face drug shortages — in August 2011, more than 40 percent of the 34 generic oncology drugs on the market were in short supply.5 There are no reliable substitutes for most of these drugs. Most are generic injectable medications that have been on the market a long time and are commonly used in hospitals, emergency rooms and cancer treatment centers.
The American Hospital Association recently reported that virtually all the community hospitals it surveyed had experienced a drug shortage in the previous six months. Nearly half had experienced a shortage of more than 20 drugs in the previous six months. Consider:
- Two-thirds of hospitals surveyed had experienced a shortage of cancer drugs.
- Eighty-eight percent were short on pain medications.
- Ninety-five percent experienced a shortage of anesthesia drugs for a surgery.
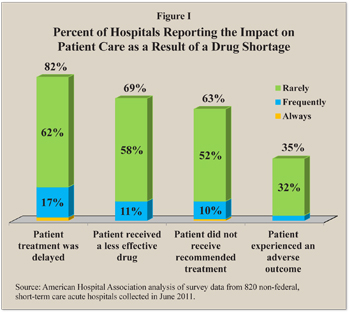
Hospitals have responded in a variety of ways, including delaying treatment, giving patients less effective drugs and providing a different course of treatment than the one recommended. Indeed, about 82 percent of hospitals surveyed reported at least occasionally delaying a treatment because of a drug in short supply. [See Figure I.]
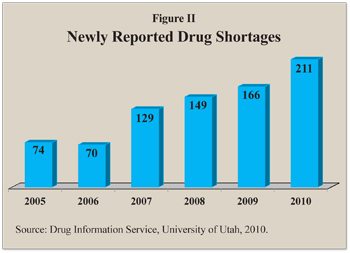
The Drug Shortage Problem Is Not New. The number of newly reported drug shortages has been growing:6
- There were 74 newly reported drug shortages in 2005.
- The number dipped slightly to 70 in 2006, then rose to 129 in 2007, 149 in 2008, 166 in 2009, and 211 in 2010.
- In mid-2011 there were about 246 shortages. [See Figure II.]
In 2005, hospitals and clinics complained to Health and Human Services Secretary Michael Leavitt that drug manufacturers and distributors were often out of certain drugs because distributors first filled their more lucrative commercial orders.7
Problem: Output Controls. About half of the shortages of injectable drugs are due to production problems.8 The FDA has stepped up its efforts to ensure that drug manufacturing processes and facilities meet its quality standards by instituting a “zero tolerance” policy. The FDA levies fines and forces manufacturers to retool both domestic and foreign facilities. For example, the FDA approves how much a drug manufacturer can produce. If a shortage develops because the FDA shuts down a competitor’s plant, a manufacturer must seek FDA approval to increase output and alter its production timetable. This slows down adjustments in production.
The Drug Enforcement Agency (DEA) has a role because minute quantities of controlled substances are often used to make other drugs.9 Its regulations are also inflexible. For example, if a shortage develops, a manufacturer that has reached its preauthorized production cap cannot respond by increasing output without DEA approval.
Problem: Medicare Part B Price Controls. Drugs that are administered by physicians —such as chemotherapy drugs or anesthesia during surgery — are paid for through Medicare Part B. Government price controls prevent the prices of these drugs from adjusting in response to shortages, increases in manufacturing costs or increases in demand. Normally, the market price of a product rises when it is short supply, attracting competing manufacturers. However, the Medicare Modernization Act (MMA) of 2003 effectively limited the amount by which the price of the drug could rise in response to market conditions over a given period.
 The 2003 law changed the way drugs administered by physicians are reimbursed under Medicare Part B. Previously, the Medicare program reimbursed drug at prices that reflected average wholesale cost. Critics claimed this arrangement was too easy to manipulate and inflated the cost. To respond to this concern, the MMA limited Medicare Part B reimbursement for physicians who administer injectable drugs to only 106 percent of the average selling price (ASP) for the past six months. Injectable drugs are more costly to produce and store than pills and capsule. If a drug is more expensive to produce than drug makers are paid for it, they won’t continue producing it. This pricing policy gives physicians an incentive to use newer patented drugs, even when older generic drugs are just as effective.
The 2003 law changed the way drugs administered by physicians are reimbursed under Medicare Part B. Previously, the Medicare program reimbursed drug at prices that reflected average wholesale cost. Critics claimed this arrangement was too easy to manipulate and inflated the cost. To respond to this concern, the MMA limited Medicare Part B reimbursement for physicians who administer injectable drugs to only 106 percent of the average selling price (ASP) for the past six months. Injectable drugs are more costly to produce and store than pills and capsule. If a drug is more expensive to produce than drug makers are paid for it, they won’t continue producing it. This pricing policy gives physicians an incentive to use newer patented drugs, even when older generic drugs are just as effective.
Additionally, because of the shortages physicians have in some cases substituted new drugs that prolong life a few months when older generic drugs that could cure a patient are unavailable. According to former FDA official Scott Gottlieb, if a shortage develops because the FDA shuts down production due to quality concerns, drug makers have little incentive to renovate manufacturing facilities to maintain production levels or accommodate needed increase in production.10
Regulations also limit the ability of drug makers to tout quality or communicate quality improvements to potential customers. This makes it difficult for drug makers to differentiate their products from competitors’ products and gain from improving quality. As a result of these and other regulations, firms cannot recoup investments they make in improving the quality of the manufacturing process.
 Problem: 340B Price Controls. The little known federal 340B drug rebate program also contributes to shortages. This program forces drug manufacturers to give discounts to outpatient clinics and hospitals that treat a high number of indigent or Medicaid patients, to Public Health Service hospitals and clinics, and to certain Federally Qualified Health Centers. Currently, the law requires manufacturers to give these facilities a 23.1 percent rebate off the average price for brand-name drugs and 13 percent for generic drugs on qualifying outpatient use.11
Problem: 340B Price Controls. The little known federal 340B drug rebate program also contributes to shortages. This program forces drug manufacturers to give discounts to outpatient clinics and hospitals that treat a high number of indigent or Medicaid patients, to Public Health Service hospitals and clinics, and to certain Federally Qualified Health Centers. Currently, the law requires manufacturers to give these facilities a 23.1 percent rebate off the average price for brand-name drugs and 13 percent for generic drugs on qualifying outpatient use.11
The Patient Protection and Affordable Care Act (ACA) — the new federal health care law — will expand the number of hospitals and clinics that qualify for rebates. The number of participating facilities has already grown from about 8,000 in 2002 to more than 14,000 by 2010. It is estimated that nearly 20,000 are eligible under the ACA.12 According to a U.S. Government Accountability Office report, nearly one-third of U.S. hospitals qualify for 340B drug discounts.13 Proposals to expand these discounts to inpatient facilities would further exacerbate the shortages — as would proposals to require rebates for Medicare enrollees who are also dual-eligible for Medicaid.
Furthermore, manufacturers that increase brand-name drug prices faster than the Consumer Price Index are required to rebate the excess amount. This means they have little incentive to purchase new equipment to maintain or improve their manufacturing processes. As a result, some drugs become less and less profitable over time.
Responses to Drug Shortages. Economics teaches that when prices are kept artificially low, shortages develop. People adjust to persistent shortages in ways that can worsen them.
Stockpiling. Buyers typically respond by hoarding drugs when the supply is uncertain. The Premier healthcare alliance, a consulting firm, explains that “drug shortages have been exacerbated by stockpiling on the part of providers,” who are trying to “protect themselves from the instability of the drug supply chain by placing orders that exceed normal requirements.”14
Occasionally, the unit rebate exceeds the average manufacturing price of a drug. Rather than requiring manufacturers to rebate more than the price of the drug, the FDA created a “penny price policy,” allowing the manufacturer to charge a minimum price of one penny per dose. When a drug is in short supply, a drug maker must restrict sales to a proportion of past purchases in order to prevent 340B-eligible facilities from hoarding or reselling drugs worth far more than the price they paid.
Black Markets. Shortages also lead to the development of black (or gray) markets, where speculators buy a drug in short supply and sell it for a much higher price. An additional problem with black markets is the potential sale of expired drugs of dubious origin.
Finally, wholesale drugs can pass from one distributor to another, resulting in multiple transfers with higher prices at each point.15 In August 2011, members of the Premier healthcare alliance report paying “gray market” prices as much as 335 percent above the approved rate.
Cascading Effects on Other Markets. Shortages in one market tend to cascade to others. In general, when hospitals cannot get a drug, they will turn to the next best alternative. But, as the Premier healthcare alliance analysis explains, when a shortage of one drug increases demand for a therapeutically similar product, the substitute may also become scarce because it “is not normally produced in quantities sufficient to meet unanticipated market needs.”16 This happened last year when a shortage of morphine lead to a shortage of the substitute painkiller hydromorphone.
Solutions. Attempts to solve drug shortages with more regulations could actually worsen the problem. Indeed, expanding the number and type of companies required to provide advance notice of impending shortages would exacerbate shortages by encouraging hospitals to hoard drugs.17 Such legislation would not make it any easier for manufacturers to avoid the problem.
Ultimately, the only way to alleviate the drug shortage is to make generic drugs more profitable. Thus, Congress should create a mechanism to reduce rebates for specific drugs in short supply. For example, injectable drugs are harder to store and involve different manufacturing processes, handling and administration than do simple tablets. Therefore, injectable drug rebates should be smaller.18 Congress should also reform regulations that reimburse physicians ASP plus a small percentage of a drug’s cost for administering it. An alternative method of reimbursement would be a fee that reflects average acquisition cost of the actual drug rather than the six-month old ASP for the entire class a drug belongs to.19
Furthermore, Emanuel and other policy analysts say injectable generic drugs should be reimbursed under Medicare Part D private drug plans rather than Part B. Competing drug plans have kept drug costs lower than they would be otherwise, and have helped maintain adequate supplies of covered drugs.
In addition, Congress should reward new investments in the manufacturing process. Limiting price increases for Part B drugs to increases in the Consumer Price Index often means that it is unprofitable to upgrade older production facilities. The federal government cannot expect firms to make necessary upgrades if profit margins do not cover costs.
Finally, regulation of production processes should be more flexible. Drug makers that want to boost production are often delayed by the approval process. For example, the FDA currently requires a triple-check verification of the manufacturing process over an extended time period.
Devon Herrick is a senior fellow with the National Center for Policy Analysis.
1 Office of the Press Secretary, “We Can’t Wait: Obama Administration Takes Action to Reduce Prescription Drug Shortages, Fight Price Gouging,” White House press release, October 31, 2011. Available at http://www.whitehouse.gov/the-press-office/2011/10/31/we-can-t-wait-obama-administration-takes-action-reduce-prescription-drug.
2 “Executive Order – Reducing Prescription Drug Shortages,” Office of the Press Secretary, The White House, October 31, 2001.
3 Ezekiel Emanuel, “Shortchanging Cancer Patients,” New York Times, August 6, 2011. Available at http://www.nytimes. com/2011/08/07/opinion/sunday/ezekiel-emanuel-cancer-patients.html.
4 “Survey Reveals 90 Percent of Anesthesiologists Experiencing Drug Shortages of Anesthetics,” Press Release, American Society of Anesthesiologists, May 9, 2011.
5 Ezekiel Emanuel, “Shortchanging Cancer Patients,” New York Times, August 6, 2011. Available at http://www.nytimes. com/2011/08/07/opinion/sunday/ezekiel-emanuel-cancer-patients.html.
6 Drug Information Service, University of Utah, 2011. Available at http://healthcare.utah.edu/pharmacy/druginfo/.
7 Letter to Michael Leavitt, Secretary of Health & Human Services, Public Hospital Pharmacy Coalition, February 17, 2005. Available at http://www.phpcrx.org/public/documents/pdfs/ IVIG_Letter.pdf.
8 Scott Gottlieb, “Drug Shortages: Why they Happen and What They Mean,” Statement before the Senate Finance Committee, United States Senate, December 7, 2011.
9 Peter Loftus, “Attention Disorder Drug Shortage Prompts Finger- Pointing,” Wall Street Journal, May 5, 2011. Available at http://online.wsj.com/article/SB1000142405274870399270457630548 2186274332.html.
10 Scott Gottlieb, “Drug Shortages: Why they Happen and What They Mean,” Statement before the Senate Finance Committee, United States Senate, December 7, 2011.
11 “Health Care Reform: 340b Drug Pricing Program,” E-ALERT, Covington Burling LLP, April 2010.
12 Stephen Barlas, “Health Care Reform Bill Expands Access to Section 340B Discounted Drugs for Hospitals,” P&T, Vol. 35, No. 11, November 2010. Available at http://www.ptcommunity. com/ptjournal/fulltext/35/11/PTJ3511632.pdf.
13 “Drug Pricing: Manufacturer Discounts in the 340B Program Offer Benefits, but Federal Oversight Needs Improvement,” Government Accountability Office, GAO-11-836, September 2011. Available at http://gao.gov/products/GAO-11-836.
14 Coleen Cherici et al., “Navigating Drug Shortages in American Healthcare: A Premier Healthcare Alliance Analysis,” Premier Inc., March 2011.
15 Jason Kane, “Drug Prices Soar as Hospital Suppliers are Forced into ‘Gray Market,’” PBS.org, August 29, 2011. Available at http://www.pbs.org/newshour/rundown/2011/08/drug-prices-soar-as-pharmacists-are-forced-into-gray-market.html.
16 Coleen Cherici et al., “Navigating Drug Shortages in American Healthcare: A Premier Healthcare Alliance Analysis.”
17 Scott Gottlieb, “Solving the Growing Drug Shortages,” Wall Street Journal, November 4, 2011.
18 Scott Gottlieb, “The Causes of Drug Shortages and Proposals for Repairing these Markets,” Statement before the Committee on Oversight and Government Reform, Subcommittee on Healthcare, United States House of Representatives, November 30, 2011.
19 Scott Gottlieb, “Drug Shortages: Why they Happen and What They Mean,” Statement before the Senate Finance Committee, United States Senate, December 7, 2011.