India is one of the top global leaders in the generic pharmaceutical drug market. The third largest drug producer in the world, India is also the second largest exporter of generic drugs to the United States, behind only Canada. India’s success in pharmaceutical manufacturing prompted President Pratibha Patil to declare, in 2010, that the next 10 years will be India’s “decade of innovation.”
However, trade relations are tense between India and the United States, because India’s lack of intellectual property rights enforcement hinders trade and pharmaceutical innovation in the two countries.
Troubles in India. In 1995, the World Trade Organization implemented the Trade-Related Aspects of Intellectual Property (TRIPS) agreement, which regulates intellectual property (IP) standards for patents and copyrights in 159 WTO member nations.2 However, though India is a member of the WTO, its patent system violates parts of the TRIPS agreement.
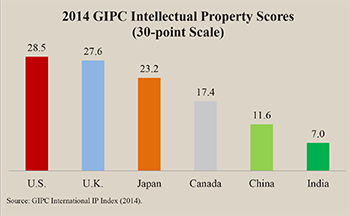
As the figure shows, the Global Intellectual Property Center’s International IP Index ranked India last out of 25 countries measured, with respect to the strength of intellectual property rights.3 The GIPC Index rates countries on their protection of patents, copyrights and trademarks. On a 30-point scale, India scored less than 7 points, compared to nearly 29 points for the United States.
Over the past two years, the Indian government has engaged in a series of policy, regulatory and legal decisions that undermine internationally recognized IP rights and are inconsistent with India’s commitments under the TRIPS agreement.
The Nexavar Case. In March 2012, India issued its first ever compulsory license for Nexavar, a kidney and liver cancer drug codeveloped by Bayer and Onyx, an American drug company. A compulsory license waives patent rules and allows other companies to make and sell a product without the consent of the company that owns the patent. Initially, under the TRIPS agreement, compulsory licensing was only to be used during public health emergencies.4
Bayer had applied for and received a patent for Nexavar in India in 2008. However, citing the high cost of the imported cancer drug and the fact that Nexavar was not manufactured locally, India’s Intellectual Property Appellate Board granted an Indian pharmaceutical company, Natco Pharma, a compulsory license to produce a generic form of the drug, shutting Bayer and Nexavar out of the Indian market. The Board granted Bayer 7 percent of net sales from Natco as required compensation for issuing the compulsory license. Yet, the drug produced by Natco is sold domestically at nearly 1/30th of the price of Nexavar.5
The Gleevec Case. In 2005, India began patenting medications in compliance with the TRIPS agreement, which requires three steps to accept a patent: required novelty, inventive step and industrial application.6 But India added a fourth element. Under Indian Patent Law section 3(d), the patent applicant must show that the drug has better therapeutic efficacy than the closest structural compound published before the patented drug.7 This fourth element prompted a legal battle with Swiss pharmaceutical company Novartis over the leukemia cancer drug Gleevec.
Novartis developed the first incarnation of Gleevec in the early 1990s. In 2006, Novartis applied for a patent on an upgraded version of Gleevec that improved the medication’s absorption into the body.8 India’s Patent Office rejected their application, arguing that Indian Patent Law section 3(d) was not satisfied because the new version of the drug was not a significant improvement over its previous incarnation. In 2011, Novartis appealed the decision to India’s Supreme Court, but the appeal was rejected in 2013.9 However, a patent for the newest version of Gleevec has been approved in more than 40 countries, including in the United States, with no resistance from authorities or other manufacturers in those countries.
The Sutent Case. U.S.-based Pfizer developed and marketed Sutent, a late-stage kidney cancer drug. The patent for Sutent was granted in 2007, but Indian generic drug companies Cipla Ltd. and Natco Pharma Ltd. opposed granting the patent. In October 2012, the Indian Patent Office revoked Sutent’s patent, claiming the drug did not prove originality in its invention under the Indian Patent Law. India’s Supreme Court reversed the Indian Patent Office’s order revoking Pfizer’s patent for Sutent for a fresh review. The Patent Office completed the second review and again revoked the Sutent patent on the same grounds of lack of inventive step. The IPAB again set aside the Patent Office’s revocation for a third review.10 For the time being, Sutent’s patent, which is approved in over 90 countries, has been reinstated.
Effects on the United States Pharmaceutical Industry. The United States is the worldwide leader in pharmaceutical innovation. Hence, U.S. pharmaceutical companies are especially affected by India’s lack of intellectual property rights enforcement. Without the certainty of patent rights, drug companies have little incentive to research and develop new drugs because the financial reward is limited, especially considering the risks. Companies spend upward of $1 billion and 10 to 15 years to develop new and innovative drugs.11 Nearly 7 out of 10 drugs developed by pharmaceutical companies fail to recoup or meet their development costs.12 The investment in the development of innovative medicines cannot be successful without the policy and regulatory conditions necessary to foster progress and promote a favorable business environment. Patents ensure that companies will earn back the money they invest in the development of new drugs.
Two-Way Trade between India and the United States. As much as U.S.-based-or-affiliated drug companies struggle to get their drugs into the Indian market, the United States is a significant market for Indian pharmaceutical companies.
For example:
Indian drug maker Lupin Ltd. is now the market leader for 24 of the 46 generic drug products it markets in the United States.13
Sun Pharmaceuticals, based in Mumbai, sells more than 57 percent of its products outside India, primarily in the United States.
Sun markets over 200 generics in the United States, with another 150 awaiting approval from the U.S. Food and Drug Administration.14
However, India shuts out U.S. pharmaceutical companies, using U.S. innovation for the benefit of their domestic companies, while Indian pharmaceutical companies profit considerably on American soil.
The U.S. Response. The Office of the U.S. Trade Representative has released its annual Special 301 report on intellectual property rights protection with U.S. trade partners.15 India has been put on the “priority watch list” for the 20th year in a row, the last category before becoming a priority foreign country. India has held the title of being on the priority watch list since 1989 – except for 1991-1993, when India was labeled a priority foreign country.16
The U.S. International Trade Commission has also begun to analyze India’s practices. Under Section 332 of the Tariff Act of 1930, the USITC initiated an investigation of India’s discriminatory and restrictive trade policies and their effects on the U.S. economy. The investigation is aimed to pressure India to reform their IP practices without resorting to trade sanctions or restrictions.
Conclusion. The United States and Indian pharmaceutical industries will play a pivotal role in the world’s access to medicine for years to come. A mutually beneficial trade agreement would prove highly profitable for both countries. India’s growing economy and drug market provides incentives for U.S. pharmaceutical companies to market innovative drugs overseas; however, in order to remain profitable, they must not be cut out of the market by domestic companies. India will not realize its “decade of innovation” without a strong trade relationship with the United States. India should work with U.S. pharmaceutical companies as the United States has worked with theirs to promote innovation while adhering to the TRIPS agreement.
Clinton Ritchey is a research associate with the National Center for Policy Analysis.
Endnotes
- Navi Radjou et al., “India’s Decade of Collaboration,” Harvard Business Review, May 31, 2011. Available at http://blogs.hbr.org/2011/05/indias-decade-of-collaboration/.
- Edward Gressor, “Global Works Foundation: TRIPS at 20,” Progressive Economy, November 19, 2013. Available at http://progressive-economy.org/2013/11/19/trips-at-20-patenting-public-health-and-an-agreement-thats-working/.
- Global Intelletual Property Center, “India: International Outlier on IP.” Available at http://www.theglobalipcenter.com/initiatives/international-advocacy/india/.
- Ibid.
- R. Jai Krishna and Jeanne Whalen, “Novartis Loses Gleevec Patent Battle in India,” Wall Street Journal, April 1, 2013. Available at http://online.wsj.com/news/articles/SB10001424127887323296504578395672582230106.
- Global Intellectual Property Center, “India: International Outlier on IP.”
- Ibid.
- “India’s Novartis Decision,” New York Times, April 4, 2013. Available at http://www.nytimes.com/2013/04/05/opinion/the-supreme-court-in-india-clarifies-law-in-novartis-decision.html?_r=0.
- R. Jai Krishna and Jeanne Whalen, “Novartis Loses Gleevec Patent Battle in India,” Wall Street Journal, April 1, 2013. Available at http://online.wsj.com/news/articles/SB10001424127887323296504578395672582230106.
- “Relief for Pfizer in Sutent patent case,” The Hindu, June 4, 2013. Available at http://www.thehindu.com/business/Industry/relief-for-pfizer-in-sutent-patent-case/article4781998.ece.
- Innovation.org, “Inside Innovation: The Drug Discovery Process.” Available at http://www.innovation.org/index.cfm/insidedrugdiscovery/Inside_Drug_Discovery
- Innovation.org, “Pharmaceutical Patents: The Value of Pharmaceutical Patents & Strong Intellectual Property Protection.” Available at http://www.innovation.org/documents/File/Pharmaceutical_Patents.pdf.
- “Quarter IV and Annual Results, FY 2012-13,” Lupin press release, May 8, 2013. Available at http://www.moneycontrol.com/livefeed_pdf/May2013/LUPIN_PR_08052013.pdf.
- “Sun Pharmaceutical Industries Ltd. equity report,” Sun Pharmaceutical Ltd, October 11, 2013. Available at http://corporate.indbankonline.com/documents/Sun%20Pharma%20Industries%20Ltd.pdf.
- Office of the United States Trade Representative, “2014 Special 301 Report,” April 2014. Available at http://www.ustr.gov/sites/default/files/USTR%202014%20Special%20301%20Report%20to%20Congress%20FINAL.pdf.
- “The USTR Special 301 Reports, 1989 to 2014,” Knowledge Ecology International. Available at http://www.keionline.org/ustr/